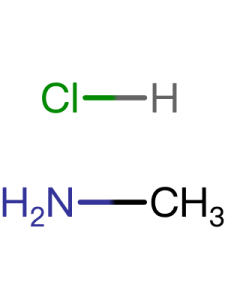
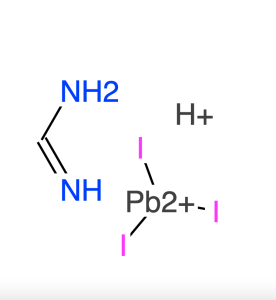
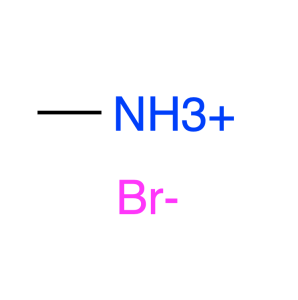| Notes | XRD & 1HNMR spectra, user instruction for enquiring |
| Store | 25℃ under N2 atmosphere |
| Packaging | 10 g, or as required in glass bottle |
| Solubility | High soluble in DMF, DMSO, et al |
| Appearance | white solid |
| Purity | 99.9% |
| Linear Formula | Br2Pb |
| Mol. Weight | 367.01 |
| CAS Number |
10031-22-8 |
| Name(EN) | PbBr2 |
| Synonym | PbBr2 |
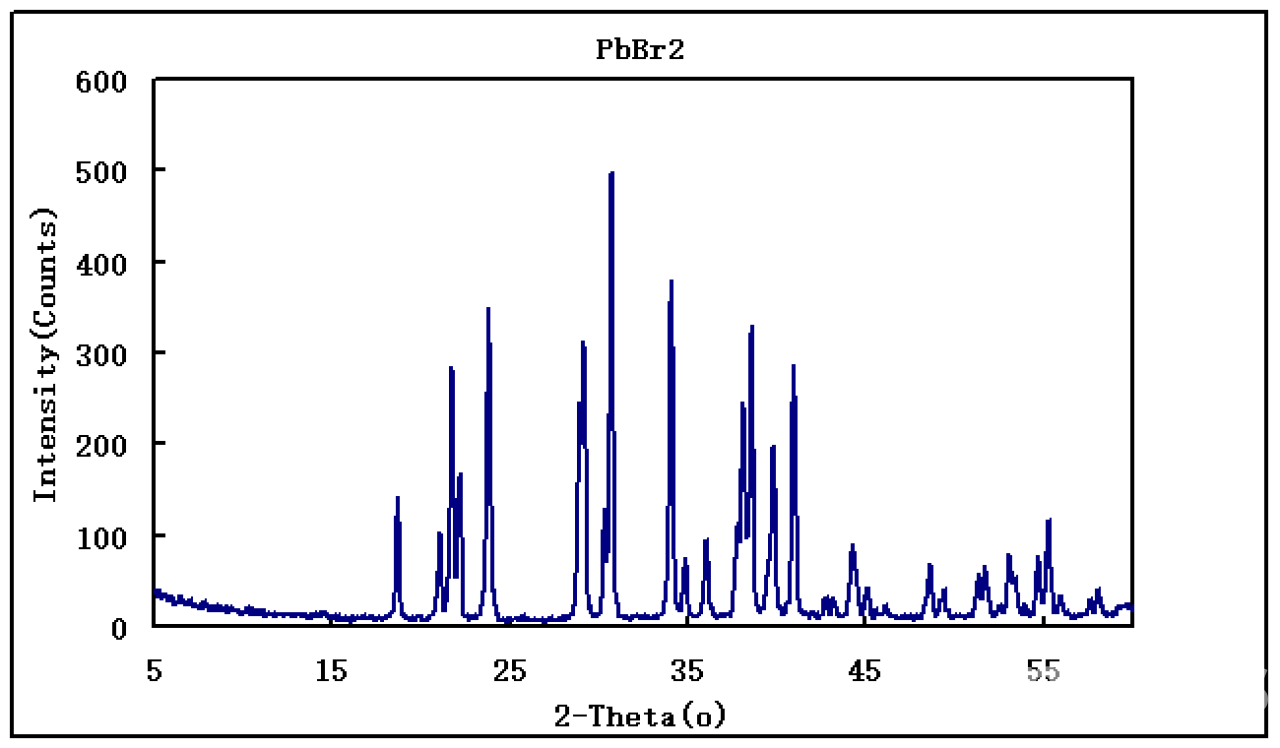
Visible-light-induced halide-exchange between halide perovskite and organohalide solvents has been studied in which photoinduced electron transfer from CsPbBr3 nanocrystals (NCs) to dihalomethane solvent molecules produces halide anions via reductive dissociation, followed by a spontaneous anion-exchange. Photogenerated holes in this process are less focused. Here, for CsPbBr3 in dibromomethane (DBM), we discover that Br radical (Br⋅) is a key intermediate resulting from the hole oxidation. We successfully trapped Br⋅ with reported methods and found that Br⋅ is continuously generated in DBM under visible light irradiation, hence imperative for catalytic reaction design. Continuous Br⋅ formation within this halide-exchange process is active for photocatalytic [3+2] cycloaddition for vinylcyclopentane synthesis, a privileged scaffold in medicinal chemistry, with good yield and rationalized diastereoselectivity. The NC photocatalyst is highly recyclable due to Br-based self-healing, leading to a particularly economic and neat heterogeneous reaction where the solvent DBM also acts as a co-catalyst in perovskite photocatalysis. Halide perovskites, notable for efficient solar energy conversion, are demonstrated as exceptional photocatalysts for Br radical-mediated [3+2] cycloaddition. We envisage such perovskite-induced Br radical strategy may serve as a powerful chemical tool for developing valuable halogen radical-involved reactions.
| Characteristic 1 | Br |
| Characteristic 2 | Pb |