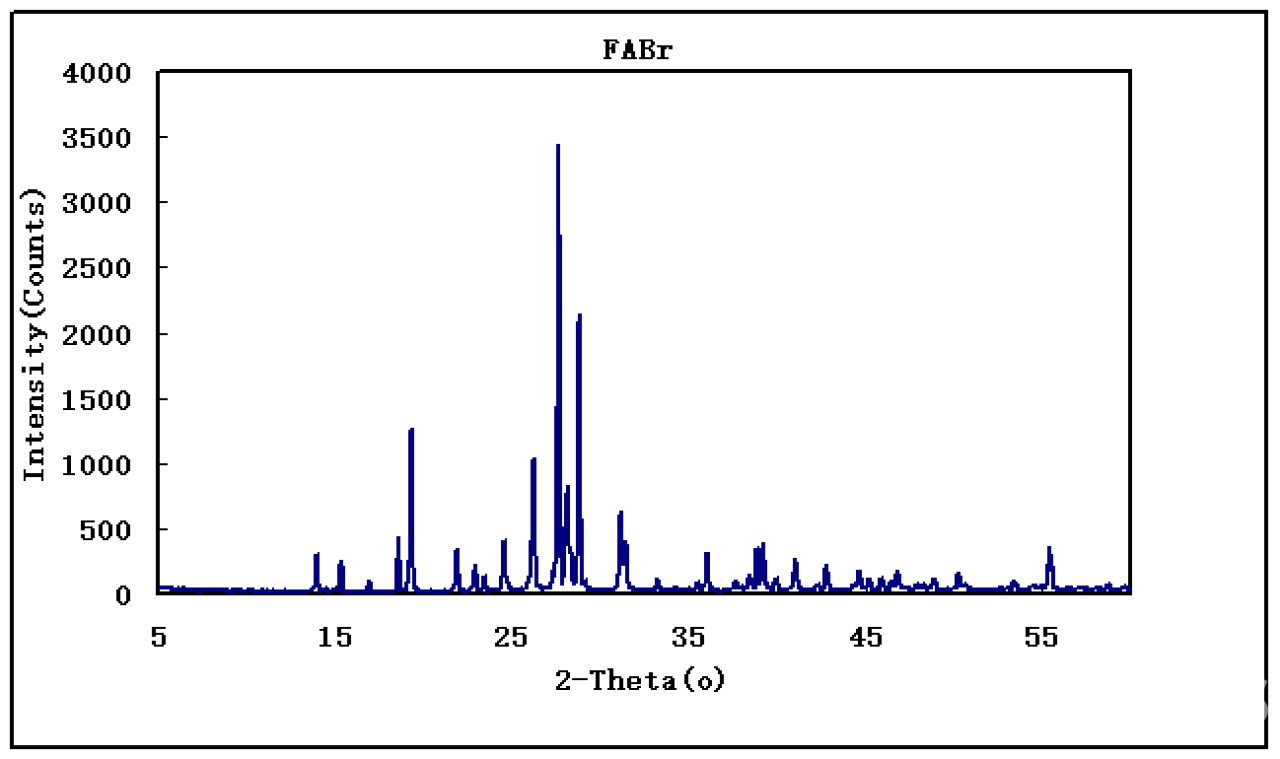
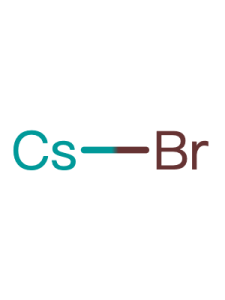| Notes | XRD & 1HNMR spectra, user instruction for enquiring |
| Store | 25℃ under N2 atmosphere |
| Packaging | 10 g, or as required in glass bottle |
| Solubility | Soluble in EtOH, DMF, DMSO, water et al |
| Appearance | white solid |
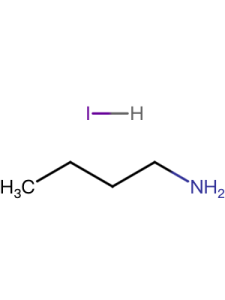
| Linear Formula | CH5BrN2 |
| CAS Number |
146958-06-7 |
| Name(EN) | FABr |
| Synonym | CH(NH2)2Br /FABr |
| Mol. Weight | 124.97 |
| Purity | 99.5% |
Abstract
Functional passivators are conventionally utilized in modifying the crystallization properties of perovskites to minimize the non-radiative recombination losses in perovskite light-emitting diodes (PeLEDs). However, the weak anchor ability of some commonly adopted molecules has limited passivation ability to perovskites and even may desorb from the passivated defects in a short period of time, which bring about plenty of challenges for further development of high-performance PeLEDs. Here, a multidentate molecule, formamidine sulfinic acid (FSA), is introduced as a novel passivator to perovskites. FSA has multifunctional groups ((Formula presented.) and -NH2) where the SO and CN groups enable coordination with the lead ions and the NH2 interacts with the bromide ions, thus providing the most effective chemical passivation for defects and in turn the formation of highly stable perovskite emitters. Moreover, the interaction between the FSA and octahedral [PbBr6]4− can inhibit the formation of unfavorable low-n domains to further minimize the inefficient energy transfer inside the perovskite emitters. Therefore, the FSA passivated green-emitting PeLED exhibits a high external quantum efficiency (EQE) of 26.5% with fourfold enhancement in operating lifetime as compared to the control device, consolidating that the multidentate molecule is a promising strategy to effectively and sustainably passivate the perovskites.
| Characteristic 1 | Br |
| Characteristic 2 | NH3 |