| Notes | XRD spectra, user instruction for enquiring |
| Store | 25℃ under N2 atmosphere |
| Packaging | 3 g, or as required in glass bottle |
| Solubility | Soluble in DMF, DMSO et al |
| Appearance | White solid |
| Purity | 99.5% |
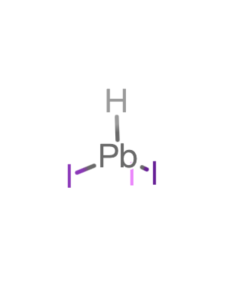
| Linear Formula | Br3HPb |
| CAS Number | 192874-55-8 |
| Mol. Weight | 447.92 |
| Stock Name | HPbBr3 |
Charge saturation and neutral substitutions in halomethanes and their group 14 analogues
Wittmaack, Bernard K.; Crigger, Chad; Guarino, Matthew; Donald, Kelling J. [Journal of Physical Chemistry A, 2011, vol. 115, # 31, p. 8743 - 8753]Abstract
A computational analysis of the charge distribution in halomethanes and their heavy analogues (MH4-nXn: M = C, Si, Ge, Sn, Pb; X = F, Cl, Br, I) as a function of n uncovers a previously unidentified saturation limit for fluorides when M ≠ C. We examine the electron densities obtained at the CCSD, MP2(full), B3PW91, and HF levels of theory for 80 molecules for four different basis sets. A previously observed substituent independent charge at F in fluoromethanes is shown to be a move toward saturation that is restricted by the low polarizability of C. This limitation fades into irrelevance for the more polarizable M central atoms such that a genuine F saturation is realized in those cases. A conceptual model leads to a function of the form [q M(n′) - qM(n)] = a[ΧA′ - ΧA] + b that links the electronegativities (Χ) of incoming and leaving atoms (e.g., A′ = X and A = H for the halogenation of MH 4-nXn) and the associated charge shift at M. We show that the phenomenon in which the charge at the central atom, qM, is itself independent of n (e.g., at carbon in CH4-nBrn) is best described as an "M-neutral substitution" - not saturation. Implications of the observed X saturation and M-neutral substitutions for larger organic and inorganic halogenated molecules and polymeric materials are identified.
| Characteristic 1 | Br |
| Characteristic 2 | Pb |