| Notes | XRD & 1HNMR spectra, user instruction for enquiring |
| Store | 25℃ under N2 atmosphere |
| Packaging | 5 g, or as required in glass bottle |
| Solubility | High soluble in EtOH, DMF, DMSO, water et al |
| Appearance | white solid |
| Purity | 99.5% |
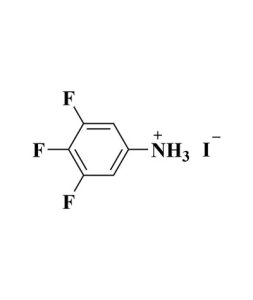
| Linear Formula | CH5IN2 |
| CAS Number |
879643-71-7 |
| Mol. Weight | 171.97 |
| Name(EN) | FAI |
| Synonym | CH(NH2)2I/FAI |
Abstract
Chemical bath-deposited SnO2 has been widely used as an electron transport layer for high-efficiency perovskite solar cells (PSCs). However, the solution-processed SnO2 has -OH groups on its surface due to proton-rich condition, which degrades the stability and efficiency of PSCs. In this study, we report on an effective way to reduce the density of surface hydroxyl groups on SnO2 and thereby improve the stability and efficiency of SnO2-based PSCs. Post-treatment of as-prepared SnO2 layer with halogen-substituted thiophenol, specifically 4-fluorothiophenol, results in substantial reduction of surface hydroxyl groups as confirmed by X-ray photoelectron spectroscopy and X-ray absorption near edge structure. Hydroxyl groups are found to act as defect sites since interfacial charge transfer and carrier lifetime of perovskite are improved by post-treatment. As a result, power conversion efficiency (PCE) is enhanced from 21% to 23% by substitution of surface hydroxyl with 4-fluorothiophenol. Moreover, a stability test for 500 h reveals that 90% of initial PCE is maintained for perovskite deposited on the surface-modified SnO2, while a significant degradation by more than 40% is observed for that formed on untreated SnO2. Pinholes generated at the perovskite/SnO2 interface are reduced by surface modification with 4-fluorothiophenol, which is responsible for the improved stability.
| Characteristic 1 | I |
| Characteristic 2 | NH3 |